Aurobindo Pharma receives EIR from USFDA for its Andhra Pradesh facility
NEW DELHI: Aurobindo Pharma Ltd on Wednesday said it has received an Establishment Inspection Report (EIR) from the US health regulator for its manufacturing facility at Pydibhimavaram in Andhra Pradesh. The company’s Unit XI at Pydibhimavaram is an API non-antibiotic manufacturing facility. It was inspected by the US Food and Drug Administration (USFDA) in February […]
Alembic Pharmaceuticals joint venture gets USFDA nod for anti-fungal drug
NEW DELHI: Alembic Pharmaceuticals Ltd on Tuesday said its joint venture, Aleor Dermaceuticals Ltd (Aleor), has received final approval from the US health regulator for its generic version of Nystatin and Triamcinolone Acetonide ointment used for the treatment of cutaneous candidiasis. Cutaneous candidiasis is an infection of the skin and nails caused by the candida […]
Cipla gets USFDA nod for inhalation product
NEW DELHI: Drug major Cipla on Wednesday said it has received final approval from the US health regulator for Arformoterol Tartrate Inhalation Solution, used to treat conditions like chronic bronchitis and emphysema, in the US market. The approved product is a generic version of Sunovion Pharmaceuticals Inc’s Brovana. The company has received final approval for […]
Zydus Cadila receives tentative approval from USFDA for Dapagliflozin Tablets
NEW DELHI: Zydus Cadila said that it has received tentative approval from the USFDA to market Dapagliflozin Tablets, 5 mg and 10 mg (US RLD: Farxiga Tablets). Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus and […]
Granules India gets USFDA approval for potassium chloride tablets
NEW DELHI: Drug firm Granules India on Thursday said it has received marketing approval from the US health regulator for Potassium Chloride extended release tablets, used for treatment of patients with hypokalemia. Hypokalemia is a condition when the potassium level in a patient’s blood is too low. The approved product is bioequivalent to the reference […]
Alembic Pharma Joint Venture gets USFDA nod for antifungal topical solution
NEW DELHI: Drug firm Alembic Pharmaceuticals on Thursday said its joint venture firm Aleor Dermaceuticals has received final approval from the US health regulator for anti-fungal Tavaborole Topical Solution. Tavaborole Topical solution is an antifungal indicated for the treatment of onychomycosis of the toenails. Aleor Dermaceuticals has received final approval from the US Food and […]
Aurobindo Pharma arm’s New Jersey unit gets warning letter from USFDA
NEW DELHI: Drug firm Aurobindo Pharma Thursday said its step-down subsidiary has received a warning letter from the US health regulator for its unit in New Jersey, US. AuroLife Pharma, a wholly-owned step-down subsidiary of the company, has received a warning letter from the United States Food and Drug Administration (USFDA) for its oral solid […]
Glenmark Pharma gets USFDA nod to market drug for relapsing multiple sclerosis
NEW DELHI: Drug major Glenmark Pharma on Wednesday said it has received final approval from the US health regulator for Dimethyl Fumarate delayed-release capsules, used for treatment of relapsing forms of multiple sclerosis in adults. The approved product is a generic version of Biogen Inc’s Tecfidera delayed-release capsules. Glenmark Pharmaceuticals Inc., USA, has been granted […]
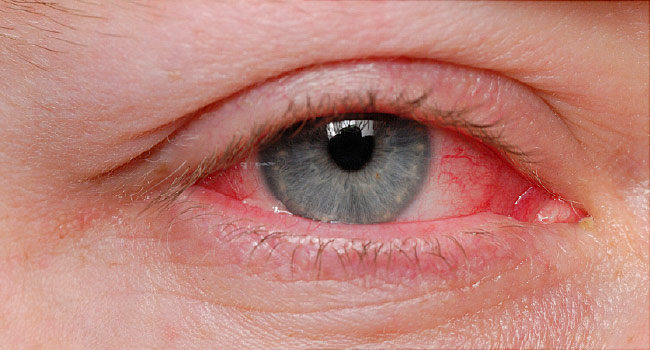
Alembic Pharma gets USFDA nod for conjunctivitis treatment drug
NEW DELHI: Drug firm Alembic Pharmaceuticals on Friday said it has received approval from the US health regulator for Azelastine Hydrochloride Ophthalmic Solution used for treatment of allergic conjunctivitis. The approved product is therapeutically equivalent to the reference listed drug Optivar Ophthalmic Solution of Mylan Specialty L P. The company has received approval from the […]
Aurobindo Pharma gets USFDA nod for infections treatment drug
NEW DELHI: Drug firm Aurobindo Pharma on Thursday said it has received final nod from the US health regulator for its Azithromycin oral suspension used for treatment of infections. The company has received final approval from the US Food and Drug Administration (USFDA) to manufacture and market Azithromycin oral suspension 100 mg /5 mL and […]